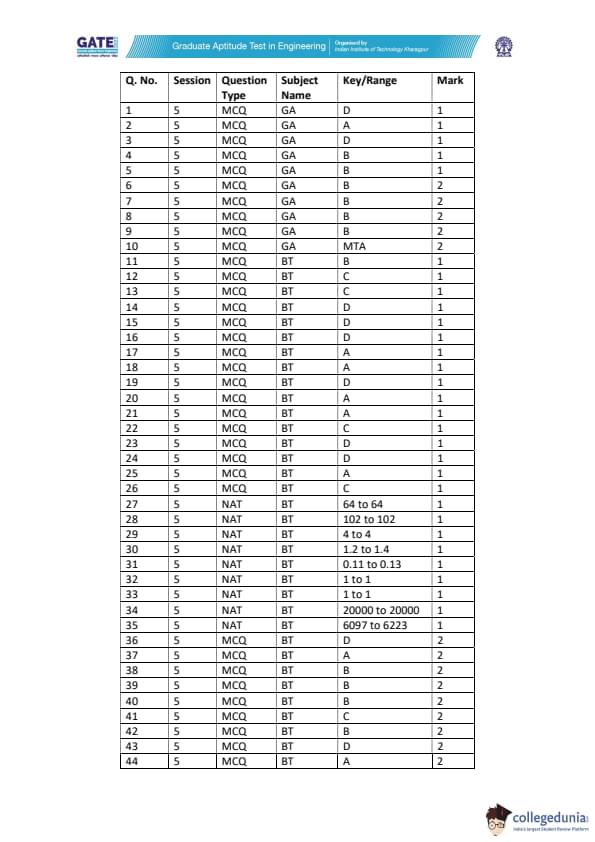
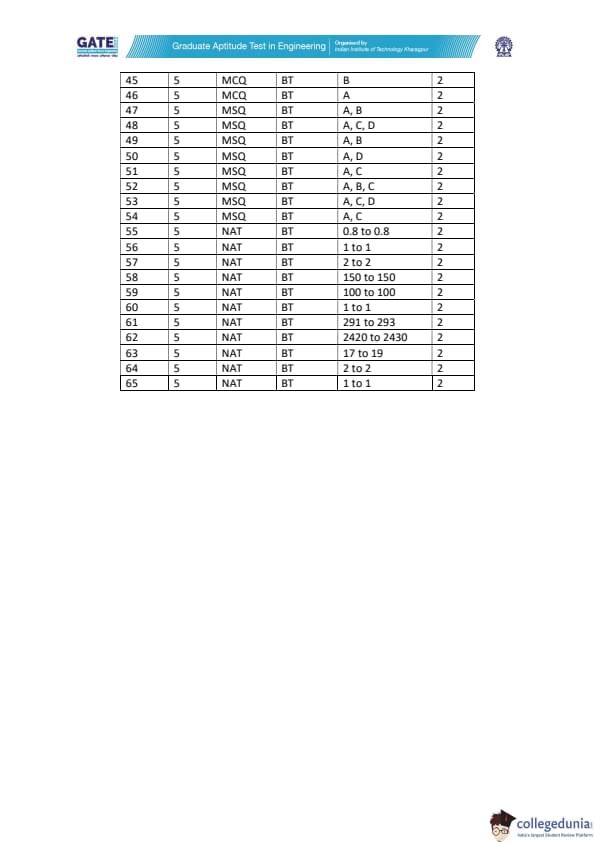
GATE 2022 Biotechnology (BT) Question Paper with Solutions PDFs are out now. GATE 2022 BT was held in February 12, 2022 by IIT Kharagpur. The duration of the exam was 180 minutes. The question paper of GATE 2022 BT was a set of 65 questions that were distributed between two parts i.e. General Aptitude and Biotechnology. 10 questions were related to General Aptitude, while the remaining 55 questions were related to core Biotechnology including Engineering Mathematics. Nearly 70% of the total weightage was carried by core Biotechnology sections.
GATE 2022 Biotechnology (BT) Question Paper with Solutions
Candidates targeting GATE can download the PDFs for GATE 2022 BT Question Paper and Solutions to know the important topics asked, and check their preparation level by solving the past question papers.
| GATE 2022 Biotechnology (BT) Question Paper | Check Solutions |

You should ______ when to say ______.
Two straight lines pass through the origin \( (x_0, y_0) = (0, 0) \). One of them passes through the point \( (x_1, y_1) = (1, 3) \) and the other passes through the point \( (x_2, y_2) = (1, 2) \). What is the area enclosed between the straight lines in the interval \( [0, 1] \) on the x-axis?
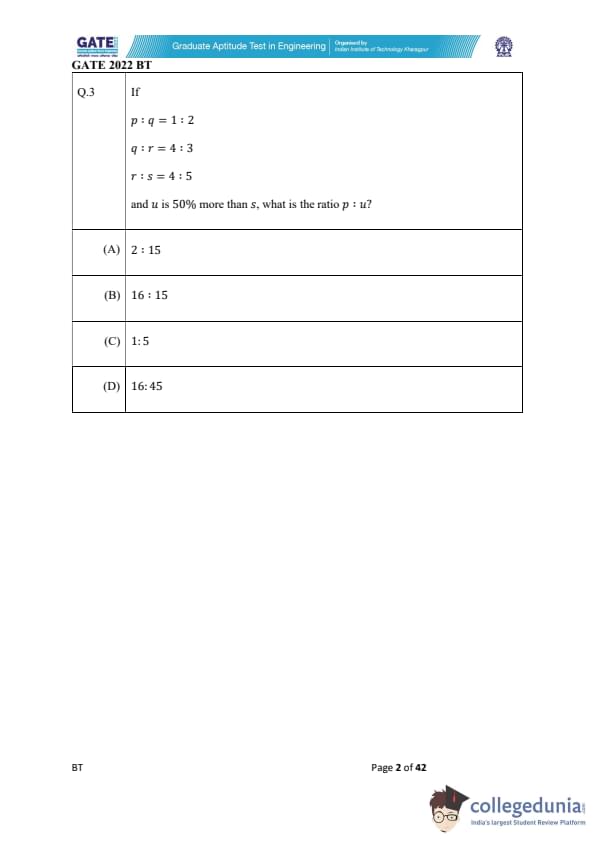
If \[ p : q = 1 : 2, \quad q : r = 4 : 3, \quad r : s = 4 : 5 \]
and \( u \) is 50% more than \( s \), what is the ratio \( p : u \)?
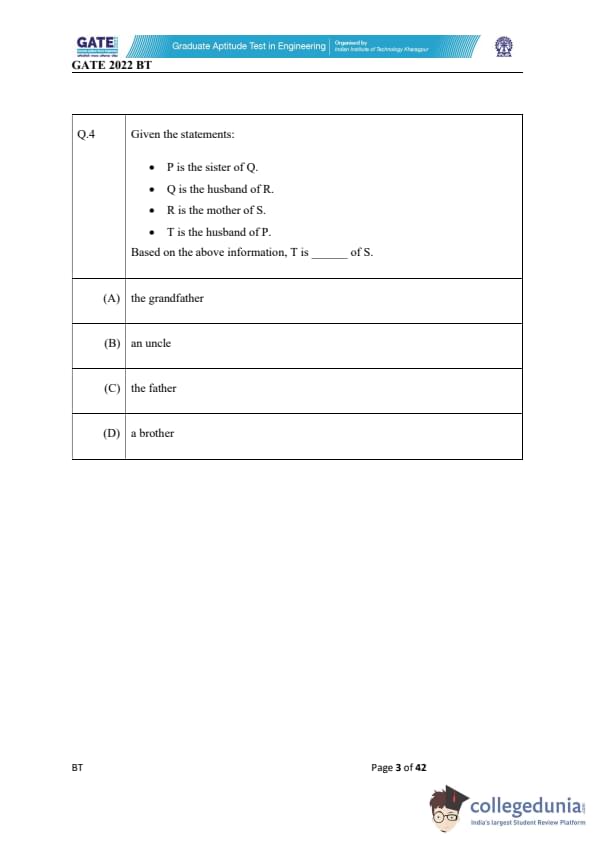
Given the statements:
P is the sister of Q.
Q is the husband of R.
R is the mother of S.
T is the husband of P.
Based on the above information, T is _________ of S.
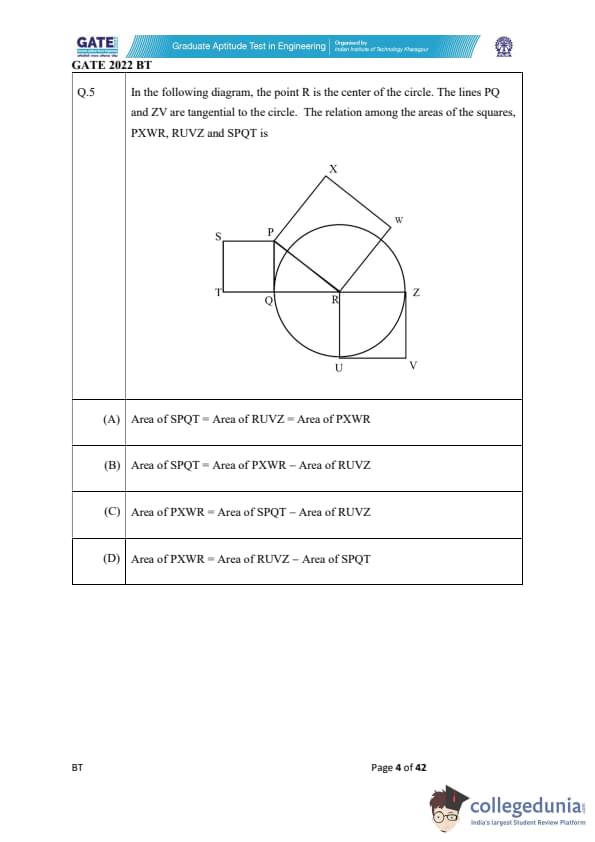
In the following diagram, the point R is the center of the circle. The lines PQ and ZV are tangential to the circle. The relation among the areas of the squares, PXWR, RUVZ and SPQT is

Healthy eating is a critical component of healthy aging. When should one start eating healthy? It turns out that it is never too early. For example, babies who start eating healthy in the first year are more likely to have better overall health as they get older.
P invested ₹ 5000 per month for 6 months of a year and Q invested ₹ x per month for 8 months of the year in a partnership business. The profit is shared in proportion to the total investment made in that year.
If at the end of that investment year, Q receives \( \frac{4}{9} \) of the total profit, what is the value of \( x \) (in ₹)?
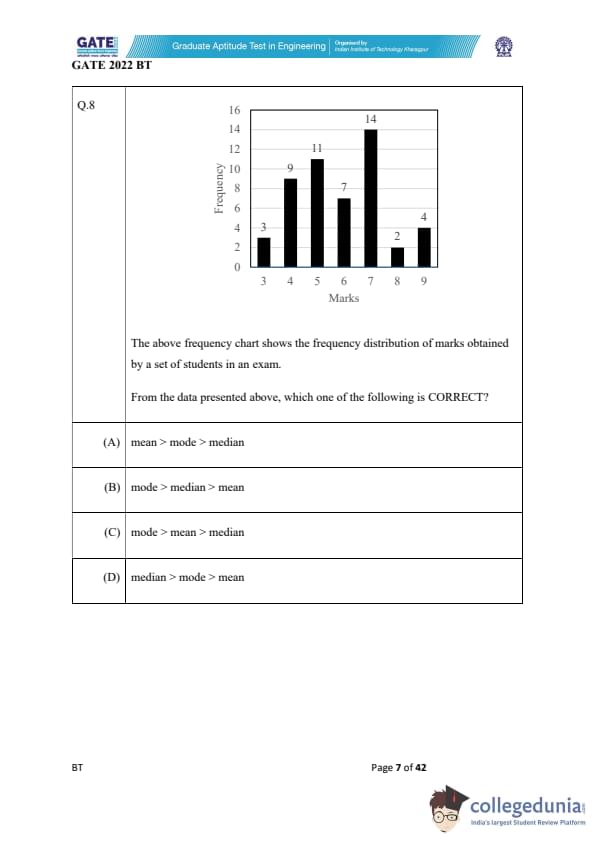
The above frequency chart shows the frequency distribution of marks obtained by a set of students in an exam.
From the data presented above, which one of the following is CORRECT?

In the square grid shown on the left, a person standing at P2 position is required to move to P5 position.
The only movement allowed for a step involves, "two moves along one direction followed by one move in a perpendicular direction". The permissible directions for movement are shown as dotted arrows in the right.
For example, a person at a given position Y can move only to the positions marked X on the right.
Without occupying any of the shaded squares at the end of each step, the minimum number of steps required to go from P2 to P5 is:

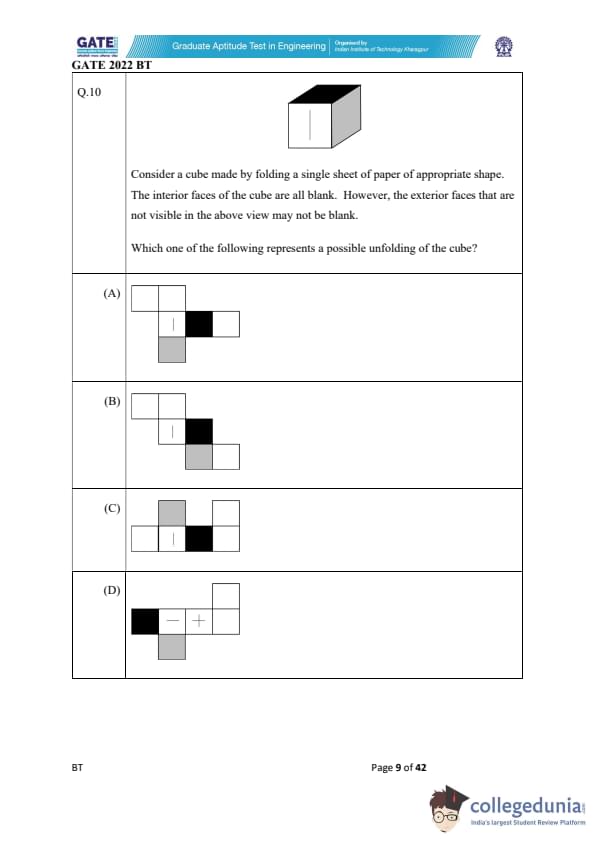
Consider a cube made by folding a single sheet of paper of appropriate shape. The interior faces of the cube are all blank. However, the exterior faces that are not visible in the above view may not be blank. Which one of the following represents a possible unfolding of the cube?


What is the order of the differential equation given below?
\[ \frac{d^2y}{dx^2} - 6x = 3x^4 - 2x^3 + 2 \]
If the eigenvalues of a \(2 \times 2\) matrix \(P\) are 4 and 2, then the eigenvalues of the matrix \(P^{-1}\) are
For a double-pipe heat exchanger, the inside and outside heat transfer coefficients are 100 and 200 W m\(^{-2}\) K\(^{-1}\), respectively. The thickness and thermal conductivity of the thin-walled inner pipe are 1 cm and 10 W m\(^{-1}\) K\(^{-1}\), respectively. The value of the overall heat transfer coefficient is _________ W m\(^{-2}\) K\(^{-1}\).
Match the media component (Column I) with its role (Column II).

The binding free energy of a ligand to its receptor protein is \( -11.5 \, kJ mol^{-1} \) at 300 K. What is the value of the equilibrium binding constant?
The overall stoichiometry for an aerobic cell growth is \( 3C_6H_{12}O_6 + 2.5 NH_3 + O_2 \rightarrow 1.5 C_a H_b O_c N_d + 3 CO_2 + 5 H_2O \).
What is the elemental composition formula of the biomass?
In binomial nomenclature, the name of a bacterial strain is written with the first letter of \underline{\hspace{2cm word(s) being capitalized.
The type of nucleic acid present in \( \lambda \)-phage is
Which of the following statements about reversible enzyme inhibitors are CORRECT?
P. Uncompetitive inhibitors bind only to the enzyme-substrate complex.
Q. Non-competitive inhibitors bind only at a different site from the substrate.
R. Competitive inhibitors bind to the same site as the substrate.
Match the component of eukaryotic cells (Column I) with its respective function (Column II).

In animal cells, the endogenously produced miRNAs silence gene expression by
Terpenoids are made of _________ units.
Match the microbial product (Column I) with its respective application (Column II).

Which of the following is NOT used for generating an optimal alignment of two nucleotide sequences?
The recognition sequences of four Type-II restriction enzymes (RE) are given below. The symbol (|) indicates the cleavage site. Identify the RE that generates sticky ends.
Among individuals in a human population, minor variations exist in nucleotide sequences of chromosomes. These variations can lead to gain or loss of sites for specific restriction enzymes. Which of the following techniques is used to identify such variations?
Assuming independent assortment and no recombination, the number of different combinations of maternal and paternal chromosomes in gametes of an organism with a diploid number of 12 is _________.
A microorganism is grown in a batch culture using glucose as a carbon source. The apparent growth yield is 0.5 g biomass / g substrate. The initial concentrations of biomass and substrate are 2 g L\(^{-1}\) and 200 g L\(^{-1}\), respectively. Assuming that there is no endogenous metabolism, the maximum biomass concentration that can be achieved is _________ g L\(^{-1}\).
The degree of reduction of lactic acid (C\textsubscript{3}H\textsubscript{6}O\textsubscript{3}) is _________.
Consider a nonlinear algebraic equation, \(x\ln x + x - 1 = 0\). Using the Newton–Raphson method, with the initial guess of \(x_{0}=3\), the value of \(x\) after one iteration (rounded off to one decimal place) is _________.
The probability density function of a random variable \( X \) is \( p(x) = 2e^{-2x} \). The probability \( P(1 \leq X \leq 2) \) (rounded off to two decimal places) is _________ .
The maximum value of the function \( f(x) = 3x^2 - 2x^3 \) for \( x > 0 \) is _________ .
The specific growth rate of a yeast having a doubling time of 0.693 h (rounded off to nearest integer) is _________ h\textsuperscript{-1}.
A fermentation broth of density 1000 kg m\textsuperscript{-3} and viscosity 10\textsuperscript{-3} kg m\textsuperscript{-1} s\textsuperscript{-1} is mixed in a 100 L fermenter using a 0.1 m diameter impeller, rotating at a speed of 2 s\textsuperscript{-1}. The impeller Reynolds number is _________.
For a pure species, the slope of the melting line \(\dfrac{dP}{dT}\) at \(-2^{\circ}\)C is \(-5.0665\times 10^{6}\) Pa K\(^{-1}\). The difference between the molar volumes of the liquid and solid phase at \(-2^{\circ}\)C is \(-4.5\times 10^{-6}\) m\(^{3}\) mol\(^{-1}\). The value of the latent heat of fusion (rounded off to nearest integer) is _________ J mol\(^{-1}\).
Which of the following conditions will contribute to the stability of a gene pool in a natural population?
P. Large population
Q. No net mutation
R. Non-random mating
S. No selection
Match the media component used in mammalian cell culture (Column I) with its respective role (Column II).

Match the cell type (Column I) with its function (Column II).

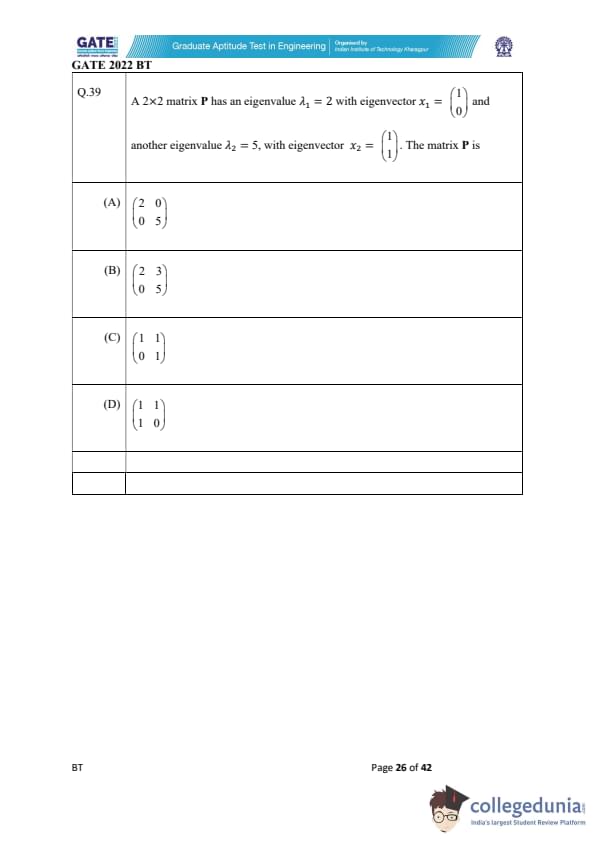
A \( 2 \times 2 \) matrix \( P \) has an eigenvalue \( \lambda_1 = 2 \) with eigenvector \( x_1 = \begin{pmatrix} 1
0 \end{pmatrix} \) and another eigenvalue \( \lambda_2 = 5 \), with eigenvector \( x_2 = \begin{pmatrix} 1
1 \end{pmatrix} \). The matrix \( P \) is
Match the stationary phase (Column I) with its corresponding chromatography technique (Column II).

Which of the following statements are CORRECT for a controller?
Which of the following are CORRECT about protein structure?
P. Secondary structure is formed by a repeating pattern of interactions among the polypeptide backbone atoms
Q. Tertiary structure is the three-dimensional arrangement of the polypeptide backbone atoms only
R. Quaternary structure refers to an assembly of multiple polypeptide subunits
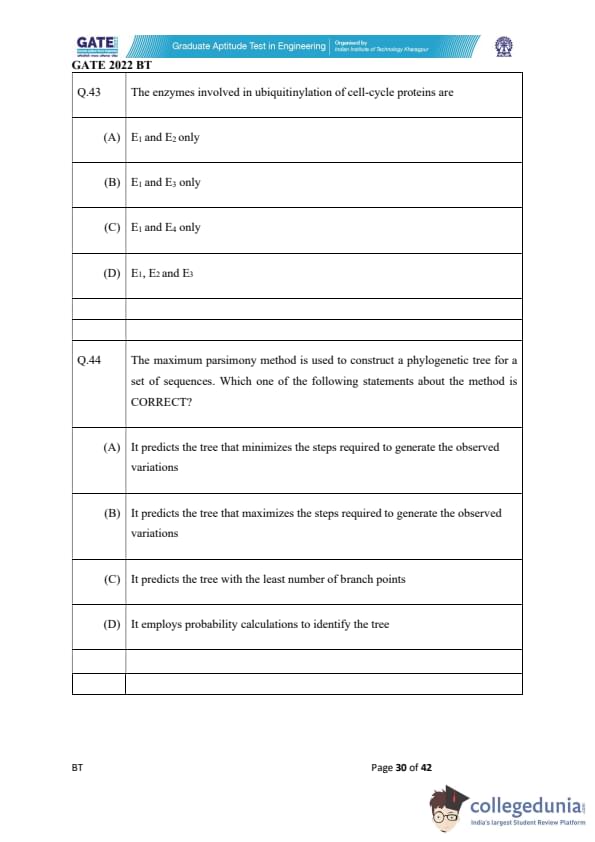
The enzymes involved in ubiquitinylation of cell-cycle proteins are
The maximum parsimony method is used to construct a phylogenetic tree for a set of sequences. Which one of the following statements about the method is CORRECT?
Which of the following spectroscopic technique(s) can be used to identify all the functional groups of an antibiotic contaminant in food?
P. Infrared
Q. Circular dichroism
R. Nuclear magnetic resonance
S. UV-Visible
Adenine can undergo a spontaneous change to hypoxanthine in a cell, leading to a DNA base pair mismatch. The CORRECT combination of enzymes involved in repairing this damage is:
Consider the ordinary differential equation \(\dfrac{dy}{dx} = f(x,y) = 2x^2 - y^2\).
If \(y(1)=1\), find \(y(1.5)\) using Euler’s implicit method:
\(y_{n+1} = y_n + h f(x_{n+1}, y_{n+1})\) with step size \(h = 0.5\).
Which of the following statements are CORRECT for an enzyme entrapped in a spherical particle?
Which of the following is(are) COMMON feature(s) for both aerobic and anaerobic bacterial cultures?
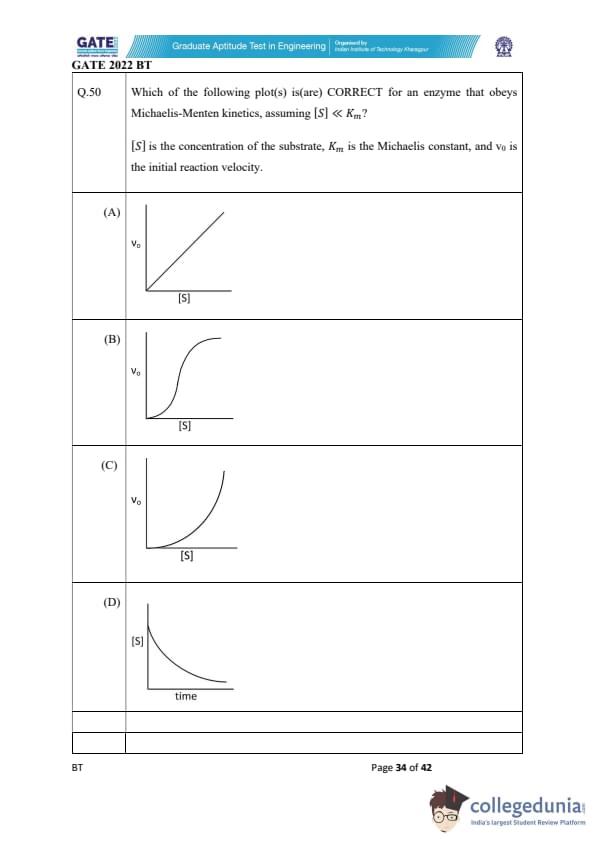
Which of the following plot(s) is(are) CORRECT for an enzyme obeying Michaelis–Menten kinetics, assuming that [S] \(\ll K_m\)?

Which statement(s) is(are) CORRECT about the lac operon of E. coli when grown in the presence of glucose and lactose?
Emerging viruses such as SARS-CoV2 cause epidemics. Which of the following process(es) contribute to the rise of such viruses?
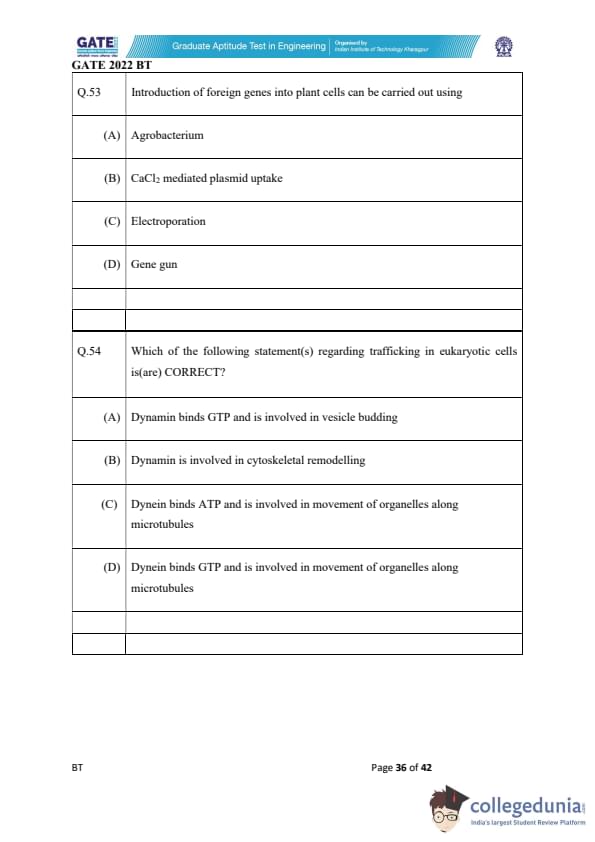
Introduction of foreign genes into plant cells can be carried out using
Which of the following statement(s) regarding trafficking in eukaryotic cells is(are) CORRECT?
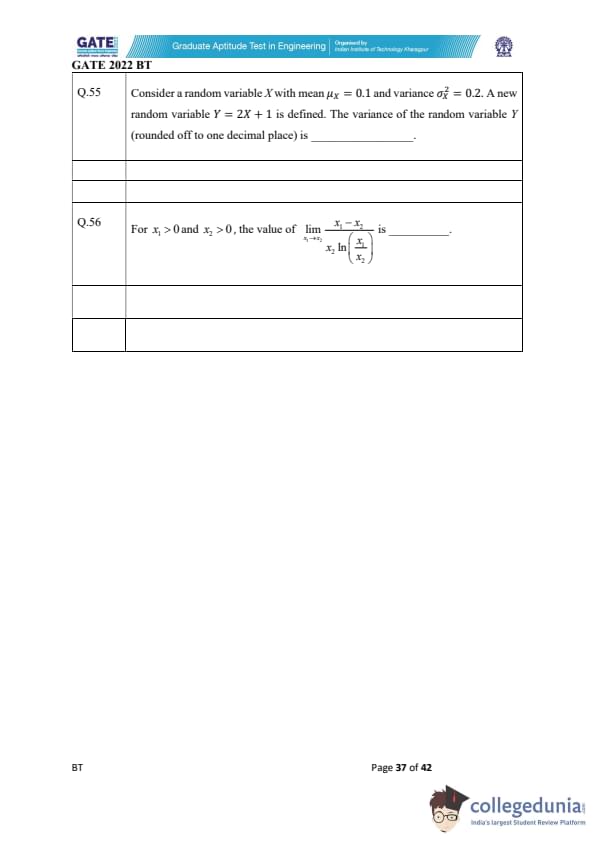
Consider a random variable \(X\) with mean \(\mu_X = 0.1\) and variance \(\sigma_X^2 = 0.2\). A new random variable \(Y = 2X + 1\) is defined. The variance of the random variable \(Y\) (rounded off to one decimal place) is _________.
For \(x_1 > 0\) and \(x_2 > 0\), the value of \(\displaystyle \lim_{x_1 \to x_2} \frac{x_1 - x_2}{x_2 \ln\left(\frac{x_1}{x_2}\right)}\) is _________.
Figure below depicts simplified metabolic and transport reactions taking place in the production of B from A in a cell. The subscript ‘i’ refers to intracellular metabolites. \(r_j\) is the \(j^{th}\) reaction flux in \(\frac{g}{(g\;dry\;mass)\;h}\). Under pseudo–steady–state condition, the following reaction fluxes are available: \(r_1 = 4,\; r_3 = 1,\; r_6 = 1\). The transport flux of B, \(r_4\), is _________ \(\frac{g}{(g\;dry\;mass)\;h}\).

A fed-batch operation is initiated by feeding substrate solution at 1 L h\textsuperscript{-1} containing 50 g L\textsuperscript{-1} substrate. The reactor initially contains 50 g biomass. The biomass yield \(Y_{X/S}^{M}\) is 0.4 \(\frac{g\;biomass}{g\;substrate}\). Assuming quasi-steady state, the maximum biomass after 5 h of feeding is _________ g.
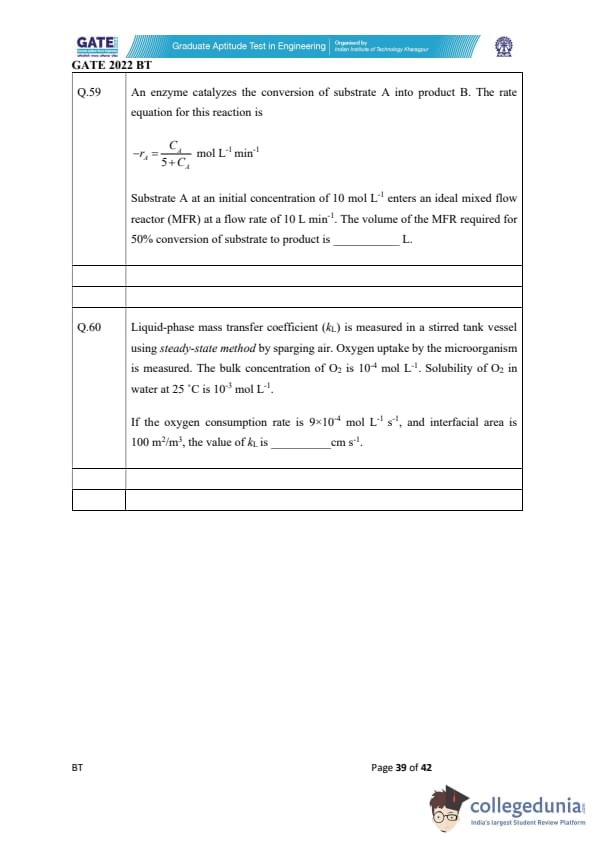
An enzyme catalyzes the conversion of substrate A into product B. The rate equation for this reaction is \[ -r_A = \frac{C_A}{5 + C_A} \;\; mol L^{-1}min^{-1} \]
Substrate A at an initial concentration of 10 mol L\(^{-1}\) enters an ideal mixed flow reactor (MFR) at a flow rate of 10 L min\(^{-1}\). The volume of the MFR required for 50% conversion of substrate to product is _________ L.
Liquid-phase mass transfer coefficient (\(k_L\)) is measured in a stirred tank vessel using steady-state method by sparging air. Oxygen uptake by the microorganism is measured. The bulk concentration of O\(_2\) is \(10^{-4\) mol L\(^{-1}\). Solubility of O\(_2\) in water at 25\(^\circ\)C is \(10^{-3}\) mol L\(^{-1}\). If the oxygen consumption rate is \(9\times10^{-4}\) mol L\(^{-1}\) s\(^{-1}\), and interfacial area is 100 m\(^2\)/m\(^3\), the value of \(k_L\) is _________ cm s\(^{-1}\).
Consider a piston–cylinder assembly as shown. The cylinder contains 1 mole of an ideal gas at 300 K, initially held at position \(z_1\). After the stopper is removed, the piston rises suddenly against atmospheric pressure (\(1.013\times10^{5}\) Pa) to a new position \(z_2\). The cylinder walls are insulated. The heat capacity at constant volume \((C_V)\) is 12.5 J mol\textsuperscript{-1 K\textsuperscript{-1. The cross-sectional area of the cylinder is \(10^{-3}\) m\textsuperscript{2. Assume the piston is weightless and frictionless. If \(z_2 - z_1 = 1\) m, the final temperature of the gas (rounded to nearest integer) is _________ K.

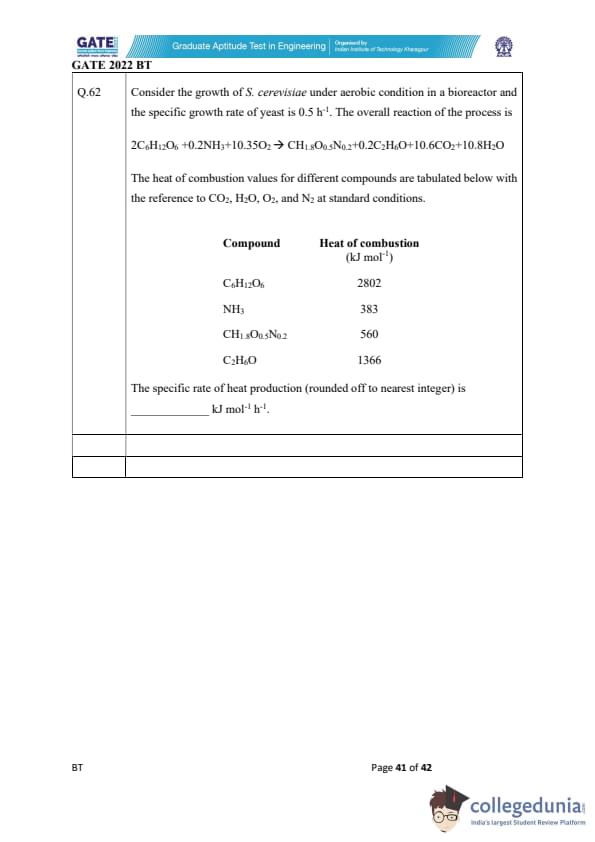
Consider the growth of S. cerevisiae under aerobic condition in a bioreactor and the specific growth rate of yeast is 0.5 h\(^{-1}\). The overall reaction of the process is \[ 2C_6H_{12}O_6 + 0.2 NH_3 + 10.35 O_2 \to CH_{1.8} O_{0.5} N_{0.2} + 0.2 C_2 H_6 O + 10.6 CO_2 + 10.8 H_2 O \]
The heat of combustion values for different compounds are tabulated below with reference to CO\(_2\), H\(_2\)O, O\(_2\), and N\(_2\) at standard conditions.

The specific rate of heat production (rounded off to the nearest integer) is _________ kJ mol\(^{-1}\) h\(^{-1}\).
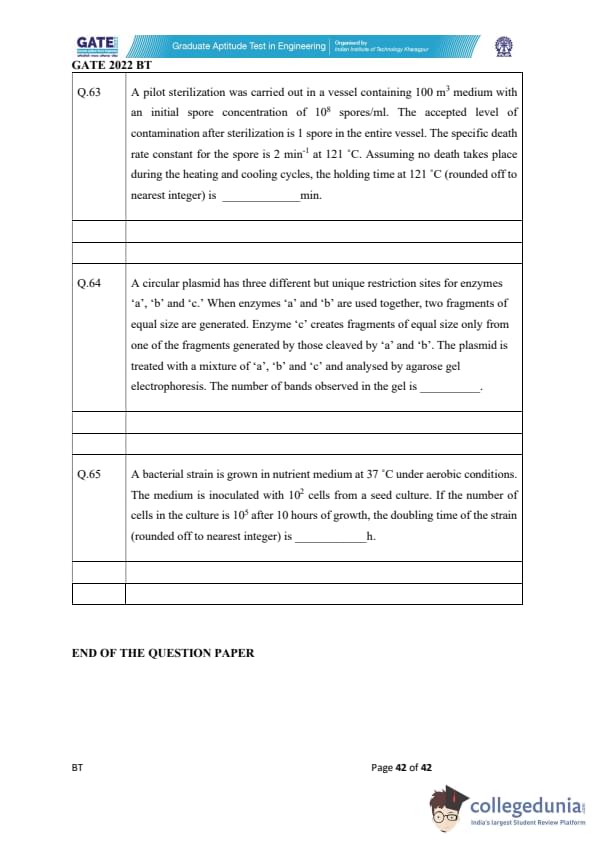
A pilot sterilization was carried out in a vessel containing 100 m\textsuperscript{3} medium with an initial spore concentration of 10\textsuperscript{8} spores/ml. The accepted level of contamination after sterilization is 1 spore in the entire vessel. The specific death rate constant for the spore is 2 min\textsuperscript{-1} at 121°C. Assuming no death takes place during the heating and cooling cycles, the holding time at 121°C (rounded off to nearest integer) is _________ min.
A circular plasmid has three different but unique restriction sites for enzymes 'a', 'b' and 'c.' When enzymes 'a' and 'b' are used together, two fragments of equal size are generated. Enzyme 'c' creates fragments of equal size only from one of the fragments generated by those cleaved by 'a' and 'b'. The plasmid is treated with a mixture of 'a', 'b' and 'c' and analysed by agarose gel electrophoresis. The number of bands observed in the gel is _________.
A bacterial strain is grown in nutrient medium at 37\(^\circ\)C under aerobic conditions. The medium is inoculated with \( 10^2 \) cells from a seed culture. If the number of cells in the culture is \( 10^5 \) after 10 hours of growth, the doubling time of the strain (rounded off to the nearest integer) is _________ h.


























Quick Links:
GATE 2022 Detailed Paper Analysis
GATE 2022 Question Paper comprises three kinds of questions i.e. MCQs (Multiple Choice Questions), MSQs (Multiple Select Questions), and NATs (Numerical Answer Type) questions. Nearly 40% of the total weightage carried by MCQs. MSQs carried the least weightage in the exam. Have a look at the below-mentioned table in order to get a thorough details of the questions-
| Question Types | Question Frequency | Carried Marks |
|---|---|---|
| No. Of 1 Mark MCQs | 21 | 21 |
| No. Of 2 Marks MCQs | 16 | 32 |
| No. Of 1 Mark MSQs | - | - |
| No. Of 2 Marks MSQs | 8 | 16 |
| No. Of 1 Mark NATs | 9 | 9 |
| No. Of 2 Marks NATs | 11 | 22 |
| Total | 65 | 100 |
All the questions were basically related to 5-6 sections. Go through the below-mentioned table, showing the details of section wise listed questions-
| Key Sections | Weightage (Questions) |
|---|---|
| General Aptitude | 10 |
| Engineering Mathematics | 10-15 |
| Plant and Animal Biotechnology | 15-20 |
| Bioprocess Engineering | 10-15 |
| Biotechnology | 20-25 |
Also Check:
GATE 2022 BT: Exam Pattern and Marking Scheme
- GATE 2022 BT asked both MCQs and NATs. It was held online via CBT mode
- As per the specified marking scheme by IIT Delhi, from the final score, ⅓ and ⅔ marks would be reduced for each wrong MCQ carried 1 and 2 marks
- Wrong attempted NATs were not supposed to bring any kind of deduction in the final achieved marks
GATE Previous Year Question Papers
| GATE 2022 Question Papers | GATE 2021 Question Papers | GATE 2020 Question Papers |
| GATE 2019 Question Papers | GATE 2018 Question Papers | GATE 2017 Question Papers |





Comments